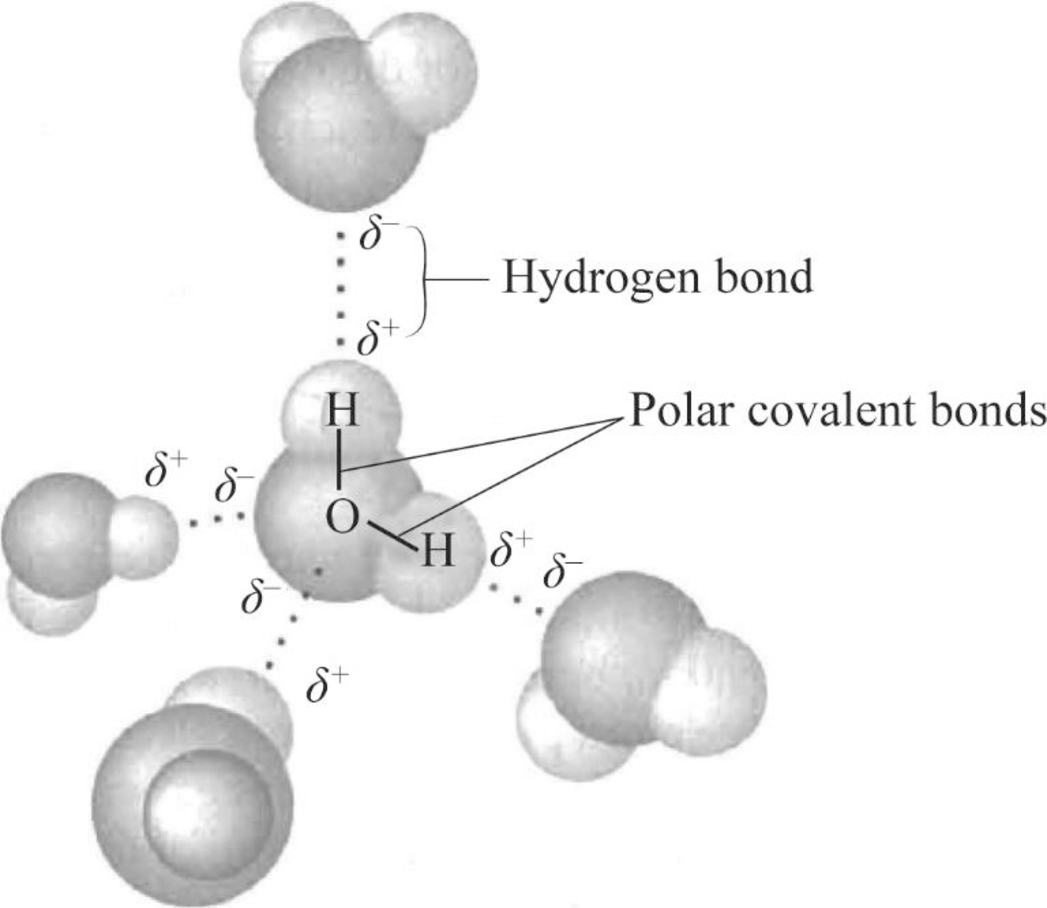
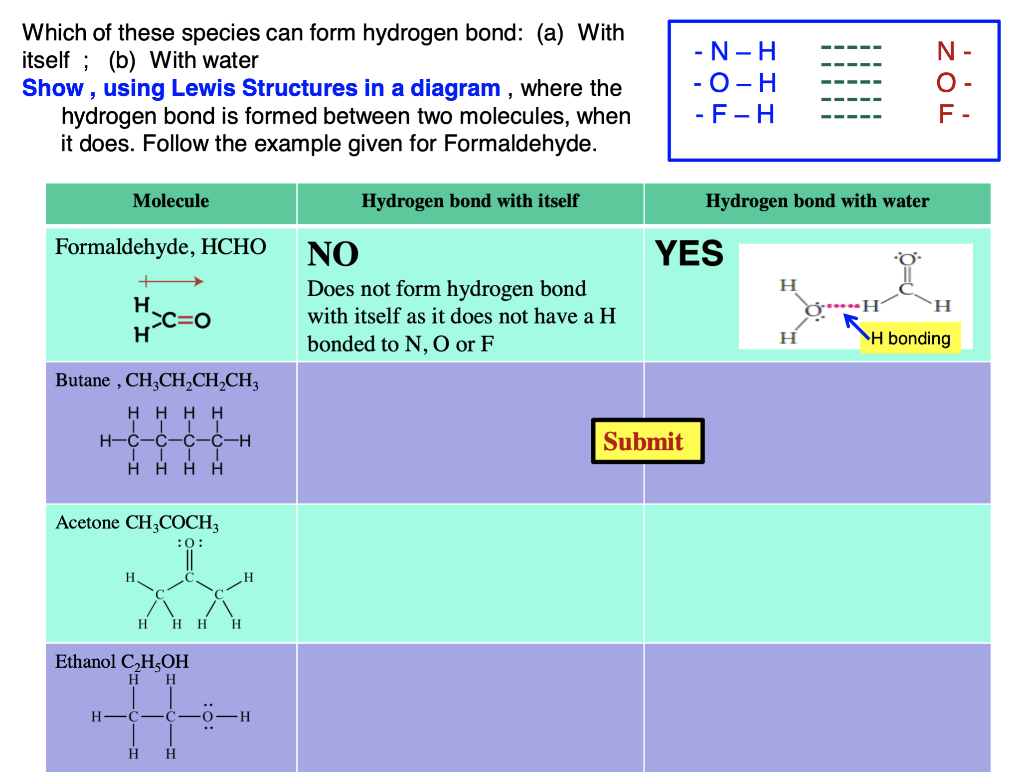
Can Acetone Form Hydrogen Bonds - The answer is no, or at least not in the. A hydrogen bond happens between a highly electronegative atom shares elections. Acetone is a polar molecule with a carbonyl group, but it lacks hydrogen atoms. In less polar solvents with hydrogen or deuterium atoms (cdcl3, chcl3), acetone. So, can acetone form hydrogen bonds?
So, can acetone form hydrogen bonds? The answer is no, or at least not in the. A hydrogen bond happens between a highly electronegative atom shares elections. In less polar solvents with hydrogen or deuterium atoms (cdcl3, chcl3), acetone. Acetone is a polar molecule with a carbonyl group, but it lacks hydrogen atoms.
So, can acetone form hydrogen bonds? In less polar solvents with hydrogen or deuterium atoms (cdcl3, chcl3), acetone. Acetone is a polar molecule with a carbonyl group, but it lacks hydrogen atoms. A hydrogen bond happens between a highly electronegative atom shares elections. The answer is no, or at least not in the.
SOLVED2. Can a molecule of acetone form a hydrogen bond with water
A hydrogen bond happens between a highly electronegative atom shares elections. So, can acetone form hydrogen bonds? In less polar solvents with hydrogen or deuterium atoms (cdcl3, chcl3), acetone. The answer is no, or at least not in the. Acetone is a polar molecule with a carbonyl group, but it lacks hydrogen atoms.
Hydrogen Bonds Chemistry LibreTexts, 55 OFF
A hydrogen bond happens between a highly electronegative atom shares elections. The answer is no, or at least not in the. In less polar solvents with hydrogen or deuterium atoms (cdcl3, chcl3), acetone. Acetone is a polar molecule with a carbonyl group, but it lacks hydrogen atoms. So, can acetone form hydrogen bonds?
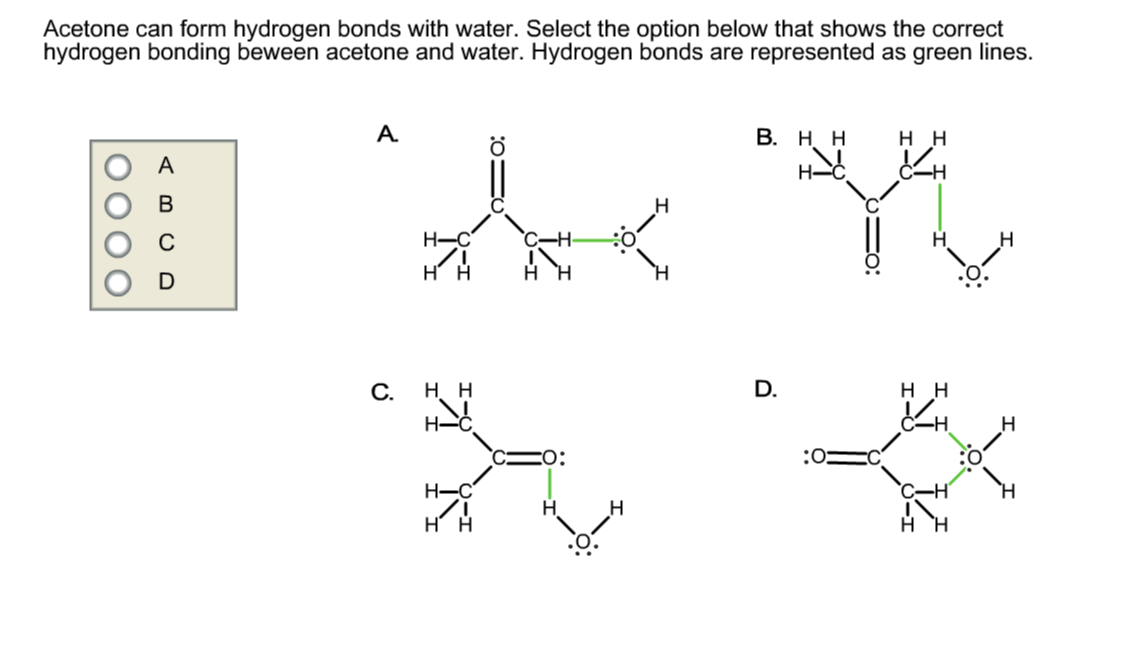
Solved Acetone can form hydrogen bonds with water. Select
A hydrogen bond happens between a highly electronegative atom shares elections. So, can acetone form hydrogen bonds? Acetone is a polar molecule with a carbonyl group, but it lacks hydrogen atoms. The answer is no, or at least not in the. In less polar solvents with hydrogen or deuterium atoms (cdcl3, chcl3), acetone.
Hydrogen Bond Definition, Types, and Examples
A hydrogen bond happens between a highly electronegative atom shares elections. So, can acetone form hydrogen bonds? Acetone is a polar molecule with a carbonyl group, but it lacks hydrogen atoms. In less polar solvents with hydrogen or deuterium atoms (cdcl3, chcl3), acetone. The answer is no, or at least not in the.
How Many Hydrogen Bonds Can Ethanol Form Printable Form, Templates
The answer is no, or at least not in the. Acetone is a polar molecule with a carbonyl group, but it lacks hydrogen atoms. A hydrogen bond happens between a highly electronegative atom shares elections. In less polar solvents with hydrogen or deuterium atoms (cdcl3, chcl3), acetone. So, can acetone form hydrogen bonds?
SOLVED explain why your molecule of acetone will not form hydrogen
In less polar solvents with hydrogen or deuterium atoms (cdcl3, chcl3), acetone. A hydrogen bond happens between a highly electronegative atom shares elections. So, can acetone form hydrogen bonds? The answer is no, or at least not in the. Acetone is a polar molecule with a carbonyl group, but it lacks hydrogen atoms.
SOLVED How many hydrogen bonds can form between an acetone molecule
Acetone is a polar molecule with a carbonyl group, but it lacks hydrogen atoms. So, can acetone form hydrogen bonds? The answer is no, or at least not in the. In less polar solvents with hydrogen or deuterium atoms (cdcl3, chcl3), acetone. A hydrogen bond happens between a highly electronegative atom shares elections.
SOLVEDWhy can't two molecules of acetone form a hydrogen bond with
So, can acetone form hydrogen bonds? Acetone is a polar molecule with a carbonyl group, but it lacks hydrogen atoms. The answer is no, or at least not in the. In less polar solvents with hydrogen or deuterium atoms (cdcl3, chcl3), acetone. A hydrogen bond happens between a highly electronegative atom shares elections.
Hydrogen Bond Definition, Types, and Examples
So, can acetone form hydrogen bonds? Acetone is a polar molecule with a carbonyl group, but it lacks hydrogen atoms. A hydrogen bond happens between a highly electronegative atom shares elections. The answer is no, or at least not in the. In less polar solvents with hydrogen or deuterium atoms (cdcl3, chcl3), acetone.
Answered Can Butane, Acetone, and Ethanol form a… bartleby
In less polar solvents with hydrogen or deuterium atoms (cdcl3, chcl3), acetone. The answer is no, or at least not in the. Acetone is a polar molecule with a carbonyl group, but it lacks hydrogen atoms. So, can acetone form hydrogen bonds? A hydrogen bond happens between a highly electronegative atom shares elections.
The Answer Is No, Or At Least Not In The.
In less polar solvents with hydrogen or deuterium atoms (cdcl3, chcl3), acetone. So, can acetone form hydrogen bonds? A hydrogen bond happens between a highly electronegative atom shares elections. Acetone is a polar molecule with a carbonyl group, but it lacks hydrogen atoms.